Answer: A)
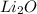
Step-by-step explanation: In order to form a neutral compound the charges must be balanced. Thus in order to neutralize the 2- charge on oxygen atom, two atoms of lithium with +1 charge must combine.
Lithium is a metal with one valence electron to form
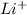 .
.
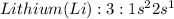
Oxygen is a non metal with 6 valence electrons to give
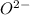
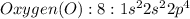
They combine to form
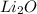 .
.