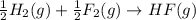Answer: A balanced chemical equation for the standard formation reaction is
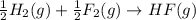
Step-by-step explanation:
A balanced chemical equation is the equation in which number of reactants equals the number number of products.
The balanced chemical equation for the formation of gaseous hydrogen fluoride is as follows.
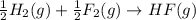
Here, in this equation half molecule of
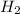 reacts with half molecule of
reacts with half molecule of
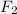 to give 1 molecule of HF.
to give 1 molecule of HF.
Thus, the sum of number of reactant molecules equals the number of product molecules. Therefore, it is a balanced equation.
Therefore, the equation is