Answer : The value of w and ΔE is, 1.24 kJ and -3170.5 kJ/mol respectively.
Explanation :
Formula used :
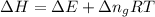
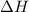 = change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol
= change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol
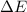 = change in internal energy
= change in internal energy
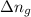 = change in moles
= change in moles
R = gas constant = 8.314 J/mol.K
T = temperature = 298 K
The balanced chemical reaction will be:
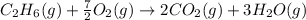
In this case:
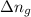 = change in moles
= change in moles
Change in moles = Number of moles of product side - Number of moles of reactant side
According to the reaction:
Change in moles = [3+2] - [1+ (7/2)]= 0.5 mole
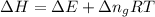
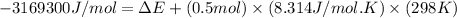
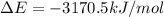
Now we have to calculate the work done.
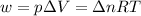
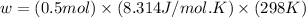
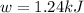
Therefore, the value of w and ΔE is, 1.24 kJ and -3170.5 kJ/mol respectively.