Answer:
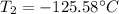
Step-by-step explanation:
Hello!
In this case, considering the Gay-Lussac's law which describes the pressure-temperature behavior as a directly proportional relationship by holding the volume as constant, we write:
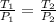
Whereas solving for the final temperature T2, we get:
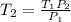
Thus, we plug in the given data (temperature in Kelvins) to obtain:

Best regards!