Answer: we started with 10 ions of Mg and 20 ions of F.
Step-by-step explanation: The given balanced equation is:
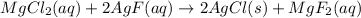
From this balanced equation, 2 formula units of AgCl are formed by 2 formula units of AgF and 1 formula unit of
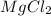 .
.
From here, the ratio between AgCl and AgF is 2:2 that is 1:1 and the ratio between AgCl and
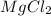 is 2:1 ,
is 2:1 ,
Since the ratio between AgCl and AgF is 1:1, 20 formula units of AgCl will be formed by 20 formula units of AgF.
The ratio between AgCl and
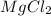 is 2:1, so 20 formula units of AgCl will be formed by 10 formula units of
is 2:1, so 20 formula units of AgCl will be formed by 10 formula units of
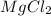 .
.
One formula unit of
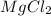 contains one ion of Mg, so 10 formula units of
contains one ion of Mg, so 10 formula units of
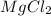 will contain 10 ions of Mg.
will contain 10 ions of Mg.
Similarly, 1 formula unit of AgF contains one ion of F, so 20 formula units of AgF will contain 20 ions of F.
So, we started with 10 ions of Mg and 20 ions of F to make 20 formula units of AgCl.