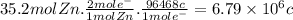Answer:
6.79 × 10⁶ c
Step-by-step explanation:
Zn can be produced from the electrolysis of an aqueous solution of a Zn salt. The reduction reaction is:
Zn²⁺(aq) + 2 e⁻ → Zn(s)
We can establish the following relations.
- 1 mol of Zn is produced when 2 moles of e⁻ are gained.
- 1 mole of e⁻ has a charge of 96468 c (Faraday's constant).
The charge needed to produce 35.2 mol of Zn is: