Answer:
2.44*10²³ Br atoms.
Step-by-step explanation:
To solve this problem we need to calculate the number of moles of CH₂Br₂ in 35.2 g.
- Molar mass of CH₂Br₂ = 12 + 1*2 + 2*79.9 = 173.8 g·mol⁻¹
35.2 g / 173.8 g·mol⁻¹ = 0.2025 mol CH₂Br₂
Now we use Avogadro's number to calculate the number of Br atoms in 0.2025 moles of CH₂Br₂, keeping in mind that there are 2 Br atoms for each CH₂Br₂ molecule:
0.2025 mol CH₂Br₂ *
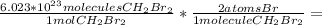 2.44*10²³ Br atoms
2.44*10²³ Br atoms