Answer: Option (c) is the correct answer.
Step-by-step explanation:
A reduction half reaction is a reaction in which there is gain of electrons.
For example,
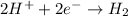 is a reduction half reaction.
is a reduction half reaction.
Whereas a reaction in which there is release or removal of electrons is known as an oxidation half reaction.
For example,
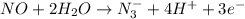 is an oxidation half reaction.
is an oxidation half reaction.
Therefore, we can conclude that out of the given options 2H ++ 2e –→ H2 is a reduction half-reaction.