Answer : The number of moles of potassium hydroxide (KOH) are needed to completely neutralize are 4.68 moles.
Explanation :
The balanced chemical reaction will be:
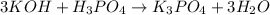
From the balanced chemical reaction we conclude that,
As, 1 mole of
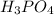 neutralizes 3 moles of KOH
neutralizes 3 moles of KOH
So, 1.56 mole of
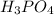 neutralizes
neutralizes
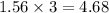 moles of KOH
moles of KOH
Thus, the number of moles of potassium hydroxide (KOH) are needed to completely neutralize are 4.68 moles.