Answer: CO is considered as a product.
Step-by-step explanation:
A general chemical equation for a combination reaction follows:
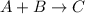
To write a chemical equation, we must follow some of the rules:
- The reactants must be written on the left side of the direction arrow.
- A '+' sign is written between the reactants, when more than one reactants are present.
- An arrow is added after all the reactants are written in the direction where reaction is taking place. Here, the reaction is taking place in forward direction.
- The products must be written on the right side of the direction arrow.
- A '+' sign is written between the products, when more than one products are present.
For the given chemical equation:
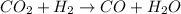
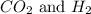 are the reactants in the reaction and
are the reactants in the reaction and
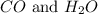 are the products in the reaction.
are the products in the reaction.
Hence, CO is considered as a product.