Answer: The correct answer is Option B.
Step-by-step explanation:
Beryllium is the 4th element of the periodic table having electronic configuration
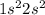 . This element belongs to Group 2 of the periodic table.
. This element belongs to Group 2 of the periodic table.
Elements that belong to same group show similar chemical properties because they have same number of valence electrons.
For the given options:
Option A: Hydrogen
This element belong to group 1 of the periodic table.
Option B: Calcium
This element belong to group 2 of the periodic table. Thus, this will show similar chemical properties to beryllium.
Option C: Lithium
This element belong to group 1 of the periodic table.
Option D: Boron
This element belong to group 13 of the periodic table.
Hence, the correct answer is Option B.