1) Write the chemical equation.
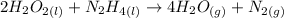
2) List the known and unknown quantities.
Sample1: H2O2.
Mass: 8.33 g.
Sample2: N2H4.
Mass: 6.48 g.
Sample3: N2
Mass of N2 produced: unknown
3) Convert mass to moles.
3.1-Convert the mass of H2O2 to moles of H2O2.
The molar mass of H2O2 is 34.01468 g/mol.
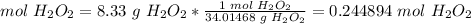
We have 0.244894 mol H2O2.
3.2-Convert the mass of N2H4 to moles of H2O2.
The molar mass of N2H4 is 32.04516 g/mol.
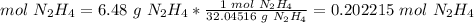
We have 0.202215 mol N2H4.
4) Limiting reactant.
4.1-How many moles of N2H4 do we need to use all of the H2O2?
The molar ratio between N2H4 and H2O2 is 1 mol N2H4: 2 mol H2O2.
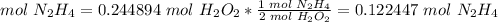
We need 0.122447 mol N2H4 and we have 0.202215 mol. We have enough N2H4. This is the excess reactant.
4.1-How many moles of H2O2 do we need to use all of the N2H4?
The molar ratio between N2H4 and H2O2 is 1 mol N2H4: 2 mol H2O2.
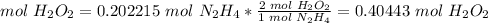
We need 0.40443 mol H2O2 and we have 0.244894 mol H2O2. We do not have enough H2O2. This is the limiting reactant.
5) Moles of N2 produced from the limiting reactant.
Limiting reactant: We have 0.244894 mol H2O2.
The molar ratio between H2O2 and N2 is 2 mol H2O2: 1 mol N2.
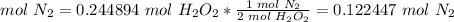
6) Convert moles of N2 to mass of N2.
The molar mass of N2 is 28.01340 g/mol.
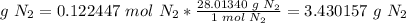
The mass of N2 produced in the reaction is 3.43 g N2.
.