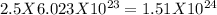Answer:
Number of atoms =
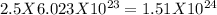
Step-by-step explanation:
In each mole of any substance there are Avagadro's number of molecule or atom.
Avagadro's number =
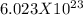
The number of moles of uranium given = 2.5 mol
The number of atoms in 2.5 moles of uranium will be
Number of atoms =