Answer:

Step-by-step explanation:
To convert from moles to grams, we must find the molar mass.
1. Molar Mass
First, identify the elements in the compound. K₂CO₃ It has potassium, carbon, and oxygen. Find these elements and their masses on the Periodic Table.
- K: 39.098 g/mol
- C: 12.011 g/mol
- O: 15.999 g/mol
Note the subscript of 2 after K and 3 after O. We must multiply oxygen's molar mass by 2, then oxygen's by 3, and add carbon.
- 2(39.098 g/mol) + 3(15.999 g/mol) + 12.011 g/mol= 138.204 g/mol
2. Convert Moles to Grams
Use the molar mass as a fraction.
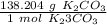
Multiply by the given number of moles: 6.2
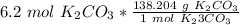
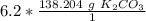
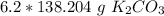
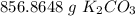
There are 856.8648 grams of potassium carbonate in 6.2 moles.