Answer : The number of moles of sodium chlorate required are, 4 moles
Solution : Given,
Moles of oxygen gas = 6 moles
The given balanced reaction is,
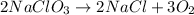
From the given balanced reaction, we conclude that
As, 3 moles of oxygen gas produces from 2 moles of sodium chlorate
So, 6 moles of oxygen gas produces from
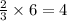 moles of sodium chlorate
moles of sodium chlorate
Therefore, the number of moles of sodium chlorate required are, 4 moles