Answer:
154° at 195 km/h
Step-by-step explanation:
The helicopter is moving south at 175 km/h, relative to the wind.
But the wind is moving east at 85 km/h, relative to the ground.
This means that the helicopter is moving south east relative to the ground.
Every hour, the helicopter will move 175 km to the south and 85 km to the east, relative to the ground.
This means that we can determine the speed and direction of the helicopter using a right triangle and simple trigonometry.
Refer to the triangle b1.
The distance traveled by the helicopter in 1 hour is denoted by d.
d is the hypotenuse of the right triangle.
Using the Pythagorean Theorem, we can calculate d to be 195 km (rounded to 3 s. f.)
Hence the helicopter is traveling at 195 km/h relative to the ground.
To calculate the direction we use,
tan (x) = opposite/adjacent = 85/175
So the angle x is,
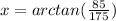 = 25.9°
= 25.9°
Relative to the North, the helicopter is moving at 180° - 25.9° = 154° (rounded to 3 s. f.)