Step-by-step explanation:
When calcium is chemically combining with oxygen then it results into the formation of chemical compound called calcium oxide.
The chemical reaction equation for this reaction is as follows.
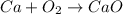
As there are not equal number of atoms on both reactant and product side. Therefore, the balanced equation will be as follows.
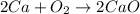
It is known that numbers present before the elements or compounds in a reaction equation are known as coefficients.
Therefore, sum of the coefficients in this equation is 2 + 1 + 2 = 5.