Answer: The final concentration of
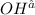 is 0.8 M.
is 0.8 M.
Explanation:
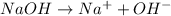

0.06 moles of NaOH will give 0.06 moles of
![[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/ud368yejy4dwvqbshzv5tjwoux4aiwn9si.png)
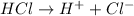
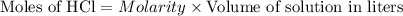
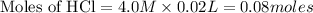
0.08 moles of HCl will give 0.08 moles of
![[H^+]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/j35v8j284apum01v8otcvhp584v9p1yjwo.png)

0.08 moles of
 will react with 0.08 moles of
will react with 0.08 moles of
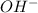 and (0.16-0.08)= 0.08 moles of
and (0.16-0.08)= 0.08 moles of
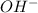 will be left in 100 ml of solution.
will be left in 100 ml of solution.
Thus Molarity of
![[OH^]-=\frac{moles}{\text {Volume in L}}=(0.08)/(0.1)=0.8M](https://img.qammunity.org/2018/formulas/chemistry/middle-school/1b9d3h9d3fgfm39sc7y70gqflrjh15uin4.png)