Answer : There are 2 paired and 3 unpaired electrons in the Lewis symbol for a nitrogen atom.
Explanation :
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot'.
The representation of valence electrons of an atom by dots around the symbol of an element is said to be the Lewis-dot symbol.
In the representation of Lewis-symbol, one electron dot must put on each side of the element symbol then paired the electrons.
The given atom is, nitrogen (N)
The electronic configuration of nitrogen atom is,
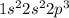
As we know that nitrogen has '5' valence electrons.
From the Lewis-dot symbol we conclude that, there are 2 paired and 3 unpaired electrons in the Lewis symbol for a nitrogen atom.
The Lewis-dot structure of nitrogen atom is shown below.