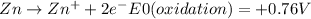Answer:
Oxidation half reaction is the reverse of the reaction depicted in the chart and the oxidation potential will have the opposite sign relative to that in the chart
Step-by-step explanation:
Redox reactions i.e. reduction-oxidation reactions generally involve transfer of electrons between two species.
Reduction half reaction involves the gain of electrons whereas oxidation half reactions involve the loss of electrons. For example
Oxidation: Loss of electrons
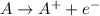
Reduction: Gain of electrons
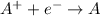
A reduction potential chart gives the standard reduction potential of various species in terms of the reduction half reaction.
For example, in the case of Zn, the standard reduction potential value (E⁰) would be:
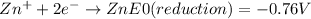
The corresponding oxidation reaction and potential would be the reverse of the above reaction: