Answer: A) -3
Step-by-step explanation:
Ions are formed when an atom looses or gain electrons.
When an atom looses its electron, it results in the formation of a positive ion which is known as cation and when an atom gains electron, it results in the formation of a negative ion known as anion.
Atomic number of nitrogen is 7 and thus has 7 electrons.
Electronic configuration of nitrogen:
![[N]:7:1s^22s^22p^3](https://img.qammunity.org/2018/formulas/chemistry/college/a6nnrc363r1x0d1sv7e551wuu95ol7tu7t.png)
In order to achieve nearest noble gas configuration of neon , it must gain 3 electrons.
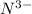 is formed by gain of 3 electrons and thus has 10 electrons.
is formed by gain of 3 electrons and thus has 10 electrons.
![[N^(3-)]:10:1s^22s^22p^6](https://img.qammunity.org/2018/formulas/chemistry/college/umubqyz50qga56oxd1mzfu4hdd7a35yavn.png)
Thus nitrogen gains 3- charge when electron configuration of the ion is similar to the nearest noble gas to the atom.