Answer:
3.18*10^24 molecules
Step-by-step explanation:
Since 1 mole of any substance consists of 6.022 × 10²³ molecules, simply do
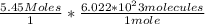 =3.18199*10^24 molecules.
=3.18199*10^24 molecules.
However, remember to include sig figs (3) and round it to 3.18*10^24 molecules
Hope this helps :)