Given :
Number of molecules of hydrogen peroxide, N = 4.5 × 10²².
To Find :
The mass of given molecules of hydrogen peroxide.
Solution :
We know, 1 mole of every compound contains Nₐ = 6.022 × 10²³ molecules.
So, number of moles of hydrogen peroxide is :
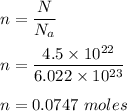
Now, mass of hydrogen peroxide is given as :
m = n × M.M
m = 0.0747 × 34 grams
m = 2.54 grams
Hence, this is the required solution.