Given that the mass of water is m1 = 500 mL at temperature T1 = 10 degrees C.
The mass of water is m2 = 100 mL at temperature T2 = 70 degrees C
We have to find the final temperature of the water mixture.
The formula to find the final temperature of the mixture is
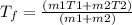
Substituting the values, the final temperature of the water will be
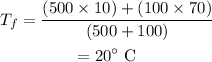
Thus, option (b) is correct.