Answer:
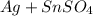
Step-by-step explanation:
Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
The elements which can easily lose or gain electrons in preference to others are chemically more reactive and are able to displace less reactive elements from their salt solutions.
The given elements are metals, thus their reactivity is based on loss of electrons.
1.
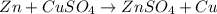
As zinc is more reactive than copper it will displace sodium from its salt solution.
2.
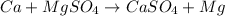
As calcium is more reactive than magnesium, it will displace magnesium from its salt solution.
3.
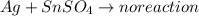
As silver is less reactive than tin, it will not be able displace tin from its salt solution.
4.

Thus as potassium is more reactive than sodium, it will displace sodium from its salt solution.