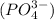Answer : The anion found in phosphoric acid is, phosphate ion
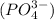
Explanation :
Phosphoric acid is a weak acid. The formula of phosphoric acid is,
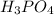 .
.
Phosphoric acid is formed when three molecules of hydrogen combine with one molecule of phosphate molecule by criss-cross method.
The formula of phosphate ion is,
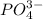 and it is a polyatomic ion composed of two or more atoms that are covalently bonded.
and it is a polyatomic ion composed of two or more atoms that are covalently bonded.
Hence, the anion found in phosphoric acid is, phosphate ion