To obtain zinc chloride, we have to react zinc and chlorine (Cl2), so let's state the chemical reaction:
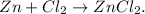
First, let's convert 0.254 grams of zinc (Zn) to moles using its molar mass which you can see in the periodic table: molar mass of Zn = 65.37 g/mol:
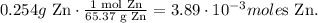
You can see in the reaction that 1 mol of Zn reacted, produces 1 mol of ZnCl2 (zinc chloride), so the molar ratios between these two compounds are 1:1, so we're producing from 3.89 x 10 ^(-3) moles of Zn, 3.89 x 10^(-3) moles of ZnCl2. Based on this value, we have to convert it to mass in grams using the molar mass of zinc chloride which is 136.17 g/mol:
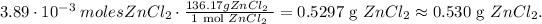
The answer is that we're producing 0.530 grams of zinc chloride from 0.254 grams of zinc reacted.