Answer: Option (a) is the correct answer.
Step-by-step explanation:
Atomic number of phosphorous is 15 and its electronic configuration is
![[Ne]3s^(2)3p^(3)](https://img.qammunity.org/2018/formulas/chemistry/college/pqp6sil1scqodtyx9s6qmfjl3qqeghgtde.png) .
.
As there are 3 valence electrons in a single phosphorous atom. Therefore, to attain stability it will readily lose three electrons and thus forms a
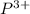 charge.
charge.
For example, in
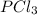 there will be sharing of electrons between the phosphorous and chlorine atom.
there will be sharing of electrons between the phosphorous and chlorine atom.
Hence, a phosphorous atom can share its three valence electrons and thus forms three covalent bonds to have a complete octet in its valence shell.