We have the reaction equation, but the equation is not balanced. We must balance it first, making sure we have the same number of atoms on each side of the reaction. We will have the balanced equation:
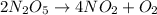
We see that for every 2 moles of N2O5, 4 moles of NO2 and one mole of O2 are formed. To convert these data to grams we will use the molar mass of each compound:
According with the equation we will have:
N2O5=2mol x 108.01g/mol=216.02g
NO2=4mol x 46.01g/mol=184.04g
O2=1molx31.998g/mol=31.998g
So, if we have the moles of the reaction. And they completely react we will have:
Answer: 184.04g of NO2 and 31.998g of O2