Step-by-step explanation:
The balanced reaction equation will be as follows.
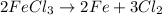
As we known that density is mass divided by volume of the substance. And, it is given that density is 7.87 g/ml and volume is 78.4 ml.
Hence, calculate the mass as follows.
Density =
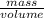
7.87 g/ml =
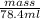
mass = 617 g
Therefore, number of moles of iron present in 617 g will be as follows.
No. of moles =
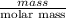
=
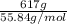
= 11.05 mol
Now, the ratio of
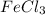 : Fe is 2:2.
: Fe is 2:2.
Hence, number of moles of
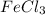 will also be equal to 11.05 mol.
will also be equal to 11.05 mol.
And, as No. of moles =
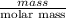
So, molar mass of
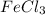 is 162.2 g/mol. Therefore, calculate the mass of
is 162.2 g/mol. Therefore, calculate the mass of
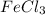 as follows.
as follows.
No. of moles =
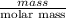
11.05 mol =
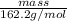
mass = 1792.31 g
Thus, we can conclude that 1792.31 g of iron (III) chloride must decompose to produce 78.4 milliliters of iron metal, if the density of iron is 7.87 g/mL.