Answer : The formal charge on the bromine atom in HBr is, 0
Explanation :
First we have to draw Lewis-dot structure of HBr.
As we know that hydrogen has 1 valence electrons and bromine has '7' valence electrons.
Therefore, the total number of valence electrons in HBr = 1 + 7 = 8
According to Lewis-dot structure, there are 2 number of bonding electrons and 6 number of non-bonding electrons.
Now we have to calculate the formal charges on bromine atom in HBr.
Formula for formal charge :
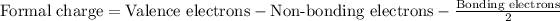
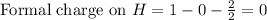
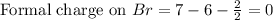
Therefore, the formal charge on the bromine atom in HBr is, 0
The Lewis-dot structure are shown below.