To find the number of atoms, we have to use Avogadro's number which says that there are 6.022 x 10 ^(23) atoms or molecules in 1 mol.
The first step is convert 90.8 grams of carbon-12 using its molar mass which is 12 g/mol:
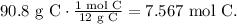
Now, we have to convert this value to the number of atoms using Avogadro's number (6.022 x 10^(23) /mol):
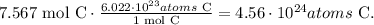
The answer is that we have 4.56 x 10^(24) atoms in 90.8 grams of carbon-12.