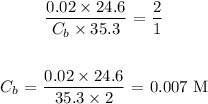Answer:
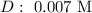
Step-by-step explanation:
Here, we want to calculate the molarity of the calcium hydroxide solution
We start by writing the equation of reaction:
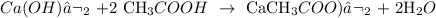
Now, we proceed to write the standardization equation:
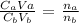
where:
Ca is the molarity of the acid which is 0.02 M
Va is the volume of the acid which is 24.6 mL
Cb is the molarity of the base which is what we want to calculate
Vb is the volume of the base which is 35.3 mL
na is the number of moles of the acid in the balanced equation which is 2
nb is the number of moles of the base in the balanced equation which is 1
Substituting the values, we have: