Answer:
N = A - Z
Step-by-step explanation:
As we know that total number of nucleons is given by sum of number of neutrons and protons.
This is known as total nucleons.
So representation of atom is given by
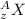
here
z = number of protons in atom
A = total number of neutrons and protons
So here if we wish to find number of neutrons then we have
N + Z = A
N = A - Z