Answer: 0.52 M
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
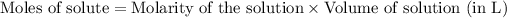 .....(1)
.....(1)
Molarity of
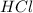 solution = 0.10 M
solution = 0.10 M
Volume of
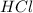 solution = 28 mL = 0.028 L
solution = 28 mL = 0.028 L
Putting values in equation 1, we get:

Molarity of
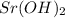 solution = 0.10 M
solution = 0.10 M
Volume of
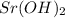 solution = 60 mL = 0.060 L
solution = 60 mL = 0.060 L
Putting values in equation 1, we get:
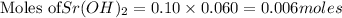
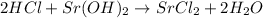
According to stoichiometry :
2 moles of
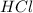 neutralize 1 mole of
neutralize 1 mole of
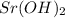
Thus 0.0028 moles of
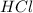 neutralize=
neutralize=
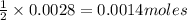 of
of
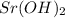
Thus (0.006-0.0014) moles = 0.046 moles of
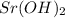 are left in 88 ml of solution.
are left in 88 ml of solution.
Concentration of
![[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/ud368yejy4dwvqbshzv5tjwoux4aiwn9si.png) will be =
will be =
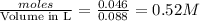
Thus the concentration of
![[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/college/ud368yejy4dwvqbshzv5tjwoux4aiwn9si.png) in the resulting solution is 0.52 M
in the resulting solution is 0.52 M