Answer: 353.936 grams of
 will be there.
will be there.
Explanation: Using Ideal gas equation, which is:
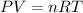
Given:
P = 2 atm
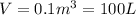 (Conversion Factor:
(Conversion Factor:
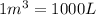 )
)
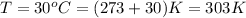 (Conversion factor = 0°C = 273 K)
(Conversion factor = 0°C = 273 K)
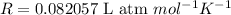 (Gas Constant)
(Gas Constant)
Putting all the values in above equation, we calculate the number of moles.
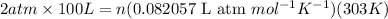
n = 8.044 moles
Now, to calculate the grams of
 , we use the formula:
, we use the formula:
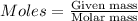
Molar mass of
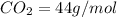
Putting the values in above equation, we get
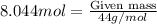
Mass of
 = 353.936 g
= 353.936 g