Chemical equilibrium is established when two opposite reactions occur simultaneously at the same rate. The equilibrium constant Kc, is the equilibrium constant and is defined as. For the following reaction:
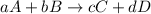
The equilibrium constant Kc will be:
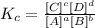
Where the letter inside the square bracket refers to the concentration of the compound.
Now, we are given the value of the equilibrium constant. To determine if the reaction is in equilibrium or not, we must find the value of Kc for the given concentrations, we have that:
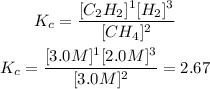
Now, the value of Kc is greater than the value of the reaction at equilibrium (Kc=0.154). This means that there is more concentration of products and for equilibrium to be reached, the reaction must shift to the reactants. This means that it will move to the left.
Answer: To the left