Answer: 1. The products of a combustion reaction do NOT include hydrogen.
2.
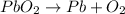 is the decomposition reaction of lead(IV) oxide.
is the decomposition reaction of lead(IV) oxide.
Step-by-step explanation:
1. Combustion reaction is a reaction in which a hydrocarbon reacts in the presence of oxygen in order to produce water and carbon dioxide.
For example,
 is a combustion reaction.
is a combustion reaction.
Thus, we can conclude that the products of a combustion reaction do NOT include hydrogen.
2. An equation is balanced when number of reactants equal the number of products.
Decomposition reaction of lead(IV) oxide is as follows.
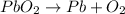
Here, the number of both reactants and products are equal. Thus, it is a balanced equation.