Answer: The element showing expanded octet is Sulfur (S).
Step-by-step explanation:
Expanded octet is shown by few elements. This rule is applied when an element can surround more than 8 electrons around them. Some of the their period elements show expanded octet. These elements are Silicon (Si), Phosphorous (P), Sulfur (S) and Chlorine (Cl).
These elements show expanded octet because they contain vacant d-orbitals.
From the given options:
Oxygen, carbon and nitrogen atoms belong to period 2 and can only surround 8 electrons around it.
Sodium belongs to period 3 and also have d-orbitals. The last electron of sodium is present in s-orbital and the energy difference of s-orbital to d-orbital is more and this transition is not favorable.
Sulfur is the only element among the options which show expanded octet because of the presence of vacant 3d-orbital. For Example: In
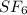 compound, the number of electrons around sulfur are 12.
compound, the number of electrons around sulfur are 12.
Hence, the element showing expanded octet is Sulfur (S).