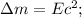Answer:
E and M are related by E= mc2, where e is energy, m is mass and c is light speed.
THEY ARE RELATED AS
When Energy is absorbed, mass is reduced.
whenever Energy is absorbed, mass is reduced.
Step-by-step explanation:
THEY ARE RELATED AS
When Energy is absorbed, mass is reduced.
whenever Energy is absorbed, mass is reduced.
E and M are related by E= mc2, where e is energy, m is mass and c is light speed. Mass m from atom must be transformed into energy to keep the nucleons together,therefore mass is called mass defects, energy is called binding energy. Technically, mass must be transformed to energy E, defined as binding energy for atomic nuclei.
When any form of energy is extracted from a process, the energy-related mass is also extracted, and thus the device loses mass. This process mass defect can be measured simply as