Answer
Number of moles of HCl = 13.48 mol
Step-by-step explanation
Given:
Moles of water = 6.74 mol
Required: Moles of HCl
Solution
Step 1: Write a balanced chemical equation
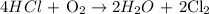
Step 2: Use the stoichiometry to calculate the moles of HCl
The molar ration between HCl and H2O is 4:2
Therefore moles of HCl = 6.74 mol x (4/2) = 13.48 mol