If we assume that nitrogen is in excess, it means that lithium is the limiting reactant, then the calculations must be made based on this reactant. Now, the equation we are given for the reaction is balanced, so we can continue with the calculations.
By stoichiometry, we can find the ratio between the reactant and the product. The Li3N to Li ratio is 2/6=1/3. So the moles of Li3N that will be formed will be:
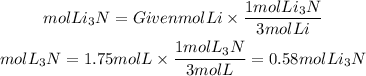
From 1.75 moles of Li can be formed 0.58 mol of Li3N