Answer:
2.97 kg.
Explanation:
We have been given that Tungsten, W-181 is a radioactive isotope with a half life of 121 days.
We will use half-life formula to solve our given problem.
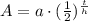 , where,
, where,
A = Final amount.
a = Initial amount,
t = Time,
h = Half life.
Upon substituting our given values in above formula we will get,
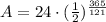
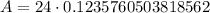
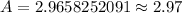
Therefore, 2.97 kg of W-181 will be left after 1 year.