Answer: Covalent compounds occur when non-metals share electrons.
Step-by-step explanation:
Covalent compound is defined as the compound which is formed by the sharing of electrons between the atoms forming a compound.
These are usually formed when two non-metals react.
For Example:
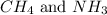 are covalent compound formed by the combination of non-metals only.
are covalent compound formed by the combination of non-metals only.
Ionic bond is defined as the bond which is formed by complete transfer of electrons from one atom to another atom. This bond is usually formed between a metal and a non-metal.
For Example: NaCl is formed by the combination of sodium and chlorine atoms.
Hence, covalent compounds occur when non-metals share electrons.