Answer: The given equation is already balanced.
Step-by-step explanation:
A balanced chemical reaction is defined as the reaction in which total mass on the reactant side is equal to the total mass on the product side.
These equations follow Law of Conservation of Mass. The mass remains conserved in a chemical reaction.
For the given chemical equation:
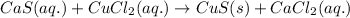
On the reactant side:
![[(40+32)+(63.5+(2* 35.5))]=206.5g/mol](https://img.qammunity.org/2018/formulas/chemistry/college/676zyugh10riv3zicl34p3slgyafjdo6gr.png)
On product side:
![[(63.5+32)+(40+(35.5* 2))]=206.5g/mol](https://img.qammunity.org/2018/formulas/chemistry/college/1chrx6mszhbst9d52kphrniuj7avght538.png)
As, the mass on reactant side is equal to the mass on product side. Hence, the equation is a balanced chemical equation.