Answer:
7.05 M
Step-by-step explanation:
- An equation can be written:
Na₃P → 3Na⁺ + P⁻³
- The formula for concentration (M) is the number of moles divided by the volume in L:
C = n / V
With the concentration and volume given by the problem, we know the number of moles of Na₃P, then with that number we can calculate the moles of Na⁺:
4.57 L * 2.35 M = 10.7395 mol Na₃P
10.7395 mol Na₃P *
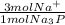 = 32.2185 mol Na⁺.
= 32.2185 mol Na⁺.
Concentration of Na⁺ = mol Na⁺ / V
[Na⁺] = 32.2185 mol Na⁺ / 4.57 L = 7.05 M