Answer:
Yes, gas is obeying the Boyle's law.
Step-by-step explanation:
Boyle's Law states that 'pressure is inversely proportional to the volume of the gas at constant temperature and number of moles'.
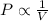 (At constant temperature and number of moles)
(At constant temperature and number of moles)
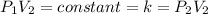
Initial volume of the gas =
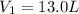
Initial pressure of the gas =
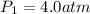
Initial value of k :
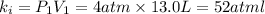
Final volume of the gas =
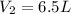
Final pressure of the gas =
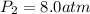
Final value of k :
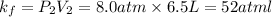
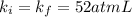
As we can see that initial value of k is equal to the final value of k which means that gas is obeying the Boyle's law.