Answer:
3 (three).
Step-by-step explanation:
Hello,
Perhaps, you are talking about the following undergoing chemical reaction:

Thus, balancing it by inspection, one obtains:
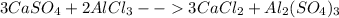
In such a way, a 3 (three) must be placed in front of the calcium chloride
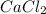 in order to balance calcium and consequently the rest of the chemical reaction.
in order to balance calcium and consequently the rest of the chemical reaction.
Best regards.