Answer : The Ne(g) effuses at a rate that is 2.828 times that of
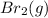 under the same conditions.
under the same conditions.
Solution : Given,
Molar mass of neon = 20 g/mole
Molar mass of bromine = 160 g/mole
Rate of diffusion : It is defined as the rate of diffusion is inversely proportional to the square root of the molar mass of the gas.
Formula used :
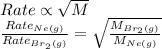
where,
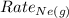 = Rate of diffusion of neon gas
= Rate of diffusion of neon gas
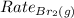 = Rate of diffusion of bromine gas
= Rate of diffusion of bromine gas
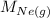 = Molar mass of neon gas
= Molar mass of neon gas
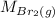 = Molar mass of bromine gas
= Molar mass of bromine gas
Now put all the given values in this formula, we get
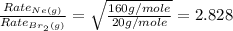
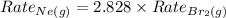
Therefore, the Ne(g) effuses at a rate that is 2.828 times that of
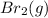 under the same conditions.
under the same conditions.