Answer : The structural formula for a hydrogen molecule and an oxygen molecule are shown below.
Explanation :
Structural formula : In the structural formula, the bonding and type of bonds which holds the atoms in molecule together are shown.
First we have to determine the Lewis-dot structure.
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule.
In the Lewis-dot structure the valance electrons are shown by 'dot' and two dots combine to form a single bond.
The given molecule is,
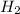
As we know that hydrogen has '1' valence electron.
Therefore, the total number of valence electrons in
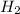 = 2(1) = 2
= 2(1) = 2
According to Lewis-dot structure, there are 2 number of bonding electrons and 0 number of non-bonding electrons. That means, there are 1 electron pairs being shared between the hydrogen-hydrogen atom.
The given molecule is,
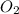
As we know that oxygen has '6' valence electron.
Therefore, the total number of valence electrons in
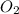 = 2(6) = 12
= 2(6) = 12
According to Lewis-dot structure, there are 4 number of bonding electrons and 8 number of non-bonding electrons. That means, there are 2 electron pairs being shared between the oxygen-oxygen atom because oxygen has ability to form double bond.